Dec 3
2019
Key Tenets of A Personalized Medicine Orchestration Platform
By Khurram Mahmood, chief technology officer, Vineti.
Personalized cell and gene therapies are expanding the frontiers of medicine. In the past two years, the FDA approved two autologous cell and gene therapies, Kymriah and Yescarta. These made history as the first commercialized therapies developed for individual patients using genetic material from the patients themselves.
This specific type of cell and gene therapy (autologous) has a unique manufacturing process that varies from that of allogeneic or traditional treatments, which can both be produced in bulk.
Alongside inspiring hope for patients, the cell and gene therapies have introduced a slew of new logistical and manufacturing challenges. The logistics around manufacturing and transporting these therapies are so new and unique, the industry is still trying to grapple with the sheer complexity of the process. Manufacturing is still very manual as automated manufacturing systems are just beginning to emerge. See Cocoon Platform from Lonza as an example on such automated platform. For therapies in pre-commercial phases, clinical trial teams are adopting agile methodologies where they build continuous feedback loops and tweak their processes based on the results from different batches of patients.
When a therapy is launched commercially, manufacturers have to on-board hundreds of hospital sites located across the globe, all with diverse IT systems and IT maturity, technology requirements, internal workflows, and national and regional regulations (which are constantly evolving). Many manufacturers are building geographically distributed facilities in an effort to more efficiently serve different regions, adding pressure to capacity management and routing capabilities.
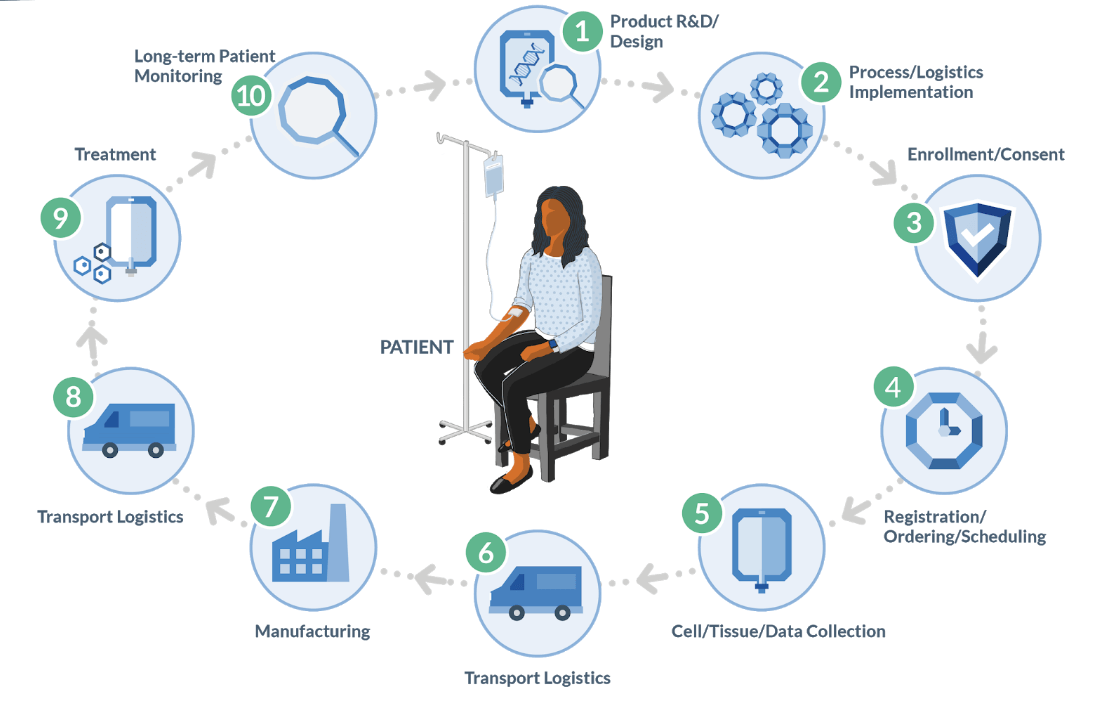
As depicted in the diagram above, the autologous process must begin at a GMP-certified collection site (most often a hospital or lab) to collect raw material (e.g. blood or tumor sample) from the patient. That is then transported to an external site to undergo a multi-step manufacturing process, creating the therapy that is then sent to an infusion site for administration to the original patient. Furthermore, the entire process is subject to timing and condition requirements.
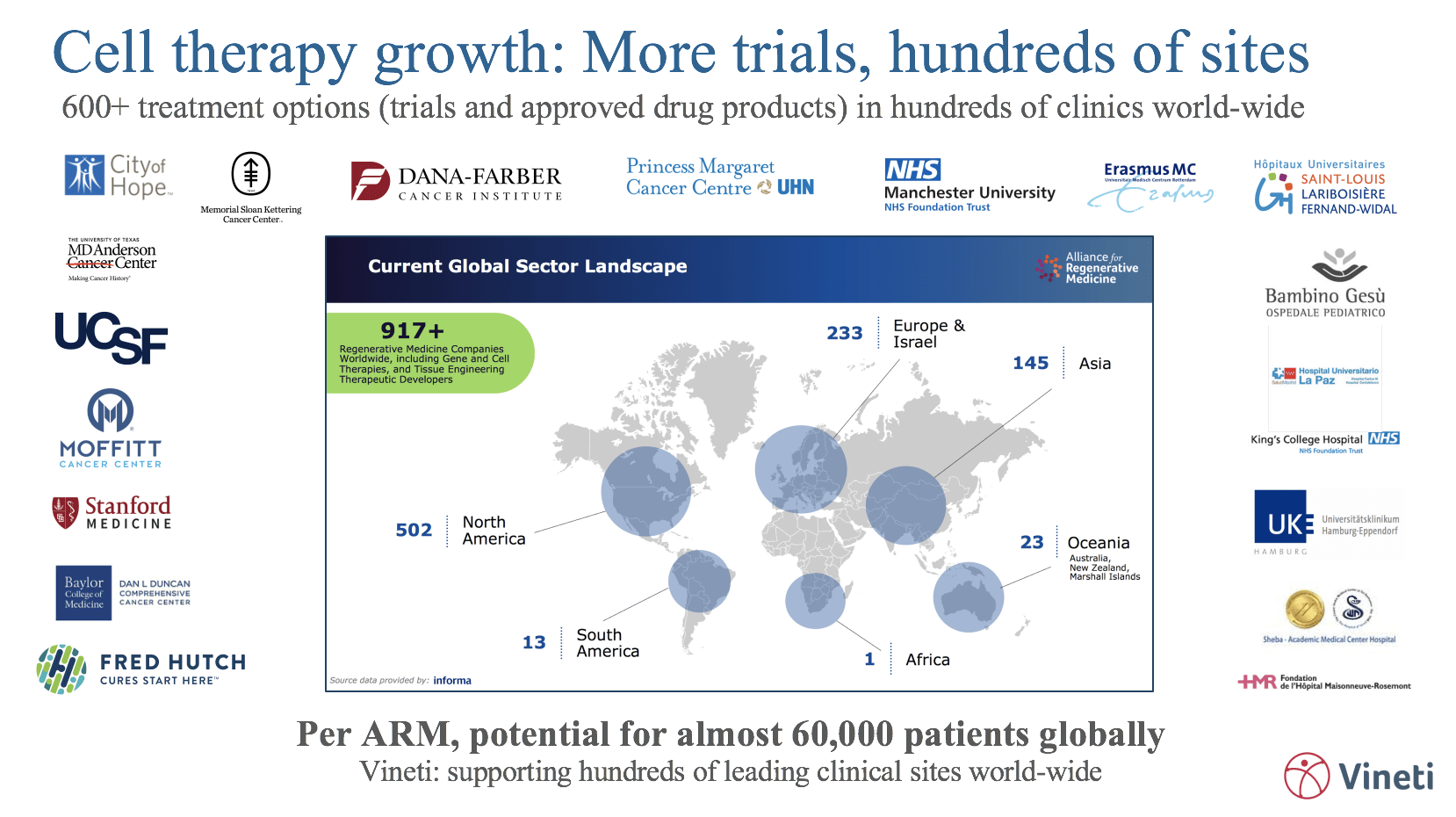
All in all, it is medicine’s most complex and risky supply chain. Currently, there are only a handful of commercial cell and gene therapies but a tsunami is coming in the next few years: The FDA is expected to approve between 10 and 20 cell and gene therapies per year between now and 2025. The time to get the right tools in place is now.
In the paragraphs below, we describe a few key tenants that a platform must possess to be able to successfully orchestrate the personalized medicine supply chain. We focus primarily on the autologous cell and gene therapy but the general principles apply to all personalized medicine.
1. Common codebase and flexibility through configuration
A platform that uses the same code base among all customers and provides flexibility through configuration is by its very nature higher quality, more feature rich, flexible and faster to change than a bespoke application built specifically for one customer.
Higher quality because the same codebase processes all transactions and thus is rigorously pressure tested – a codebase that has processed hundreds of thousands of patients is always going to be more robust than the one processing its first patient.
Flexible because such a platform has to, by necessity, enable patterns and then allow for those patterns to be configured for several different use cases. As such it is not tied to one use case and can easily and quickly be re-configured to support other use cases as the market needs change.