Dec 3
2019
Key Tenets of A Personalized Medicine Orchestration Platform
By Khurram Mahmood, chief technology officer, Vineti.
Personalized cell and gene therapies are expanding the frontiers of medicine. In the past two years, the FDA approved two autologous cell and gene therapies, Kymriah and Yescarta. These made history as the first commercialized therapies developed for individual patients using genetic material from the patients themselves.
This specific type of cell and gene therapy (autologous) has a unique manufacturing process that varies from that of allogeneic or traditional treatments, which can both be produced in bulk.
Alongside inspiring hope for patients, the cell and gene therapies have introduced a slew of new logistical and manufacturing challenges. The logistics around manufacturing and transporting these therapies are so new and unique, the industry is still trying to grapple with the sheer complexity of the process. Manufacturing is still very manual as automated manufacturing systems are just beginning to emerge. See Cocoon Platform from Lonza as an example on such automated platform. For therapies in pre-commercial phases, clinical trial teams are adopting agile methodologies where they build continuous feedback loops and tweak their processes based on the results from different batches of patients.
When a therapy is launched commercially, manufacturers have to on-board hundreds of hospital sites located across the globe, all with diverse IT systems and IT maturity, technology requirements, internal workflows, and national and regional regulations (which are constantly evolving). Many manufacturers are building geographically distributed facilities in an effort to more efficiently serve different regions, adding pressure to capacity management and routing capabilities.
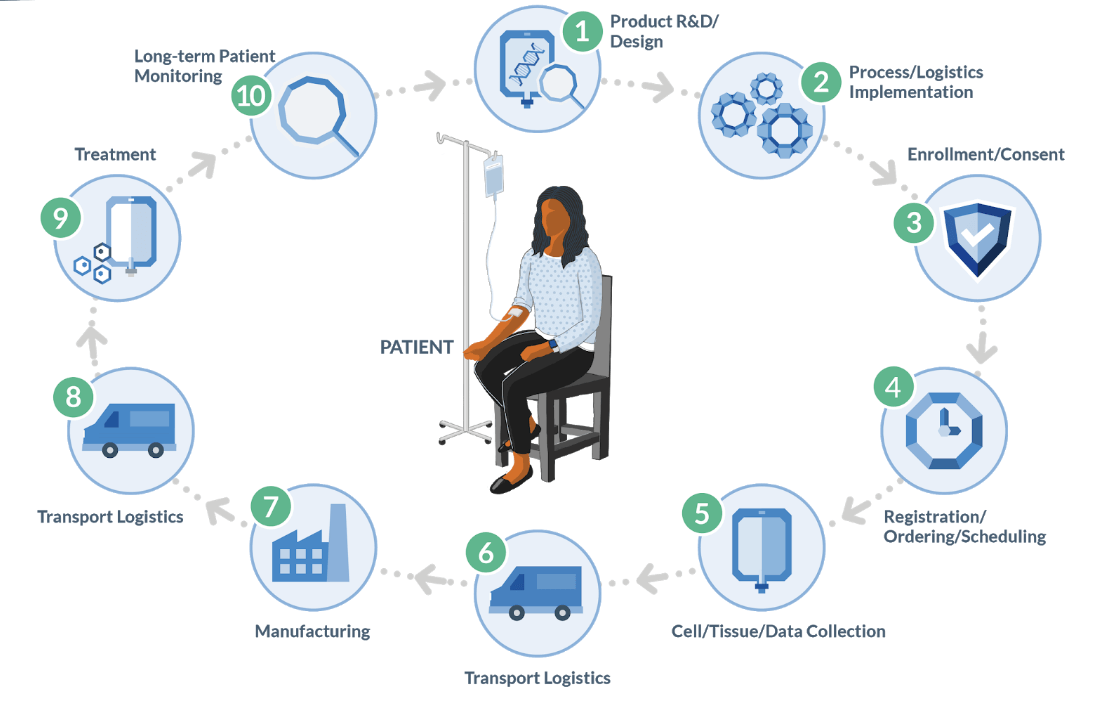
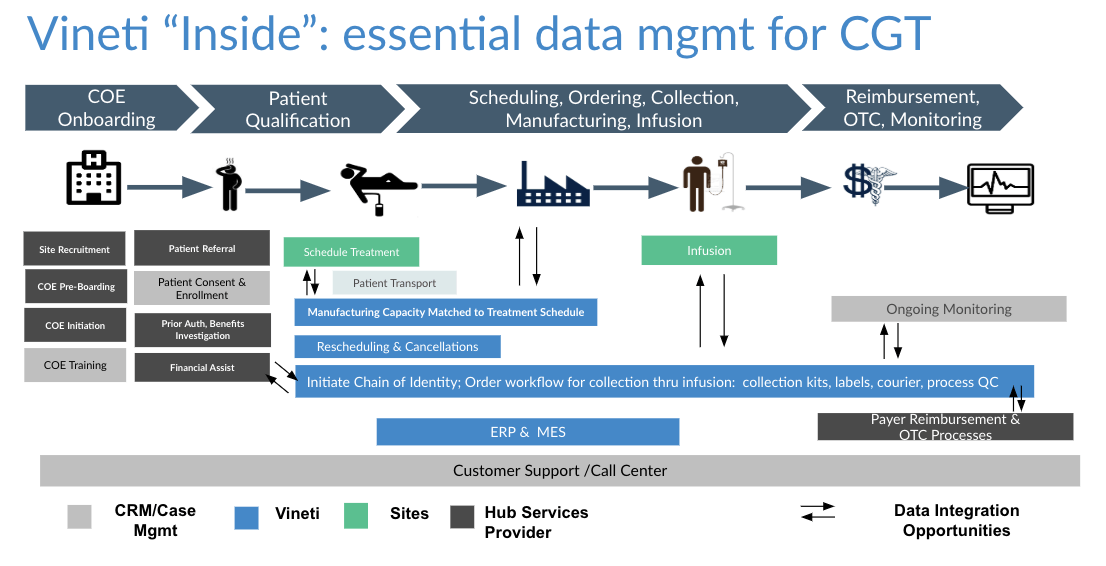
As depicted in the diagram above, the autologous process must begin at a GMP-certified collection site (most often a hospital or lab) to collect raw material (e.g. blood or tumor sample) from the patient. That is then transported to an external site to undergo a multi-step manufacturing process, creating the therapy that is then sent to an infusion site for administration to the original patient. Furthermore, the entire process is subject to timing and condition requirements.
All in all, it is medicine’s most complex and risky supply chain. Currently, there are only a handful of commercial cell and gene therapies but a tsunami is coming in the next few years: The FDA is expected to approve between 10 and 20 cell and gene therapies per year between now and 2025. The time to get the right tools in place is now.
In the paragraphs below, we describe a few key tenants that a platform must possess to be able to successfully orchestrate the personalized medicine supply chain. We focus primarily on the autologous cell and gene therapy but the general principles apply to all personalized medicine.
1. Common codebase and flexibility through configuration
A platform that uses the same code base among all customers and provides flexibility through configuration is by its very nature higher quality, more feature rich, flexible and faster to change than a bespoke application built specifically for one customer.
Higher quality because the same codebase processes all transactions and thus is rigorously pressure tested – a codebase that has processed hundreds of thousands of patients is always going to be more robust than the one processing its first patient.
Flexible because such a platform has to, by necessity, enable patterns and then allow for those patterns to be configured for several different use cases. As such it is not tied to one use case and can easily and quickly be re-configured to support other use cases as the market needs change.
Take the collection and logistics processes as examples where raw material such as blood is collected from the patient at a hospital site and transported to a manufacturing facility. Each autologous therapy has a different workflow for collection, logistics and manufacturing. In addition, each therapy requires the HCPs to input different sets of data. A platform would enable standard and custom data fields, allow them to be assembled into steps that are then strung together to form complete workflows. Then the same platform would have to provide a number of configurations for managing scheduling and logistics. Scheduling based on logistics is NP-hard problem with so many permutations that they cannot be modeled at configuration time. That’s why the platform must support a combination of rule-based and machine learning techniques that we describe here.
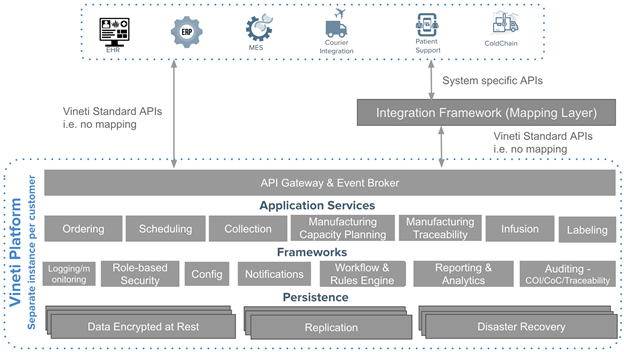
This approach would support a large number of unique requirements while keeping each customer’s configurations isolated and hidden from another. And as the platform evolves to support more patterns out-of-the-box, all customers would benefit from that innovation for free. This is a proven approach adopted by the leading enterprise software companies.
2. Accessible and intuitive
Making it easy and intuitive is key. When onboarding a collection site, therapy manufacturers train and qualify the site’s HCPs, including how to use the system to perform SOPs. Since the HCPs use many disparate systems to do their work, they tend to forget what they learned. Some existing systems in the personalized medicine space have been built for a ‘back-office’ admin user as they have evolved from legacy supply chain platforms. Many HCPs struggle and often get stuck when trying to use these systems and have to call the manufacturer’s support teams for help. This not only slows down the process but also poses a risk of error.
There are following three requirements that user interface for a personalized medicine platform must meet.
Firstly, a user interface that is to be used by a nurse at a hospital (as an example) has to be conceived, designed and built to match the mental model of the HCP persona so that it is simple, intuitive and productive. It has to take its cues from consumer applications and not be last-minute bolt-on on an existing back-office platform.
Secondly, the user interface needs to have the ability to tailor itself to suit the needs of every user profile. And then learn from how the product is being used and refine it by continuously tweaking the interface. I would contend that even the back-office users wouldn’t mind a more intuitive user interface than the one that they have been historically subjected to by the traditional enterprise systems. This focus on intuitive user interfaces that adapt to their user’s needs is the hallmark of cloud based configurable platforms and is very hard to do in custom or on-prem applications.
Lastly, a web-based platform that is available across common browsers on desktop and mobile channels ensures universal accessibility for all users and is a must-have for the personalized medicine industry.
3. Flexible – easy and quick to adapt
Heterogeneity, global reach, and change are the hallmarks of personalized medicine. A platform must offer advanced capabilities for configurability and adaptation in order to keep pace with changes in the industry. This includes deep customization including but not limited to screens, data acquisition, workflows, rules and application programming interfaces (APIs). This ability to support such deep customizations through a configurable framework is the secret sauce for such platforms. It is also the key source of complexity that must be managed and controlled very carefully otherwise every upgrade becomes a nightmare. Cloud platform pioneers have achieved this level of flexibility in general purpose horizontal platforms.
As depicted in the diagram above, the platform must support multiple levels of configuration to allow workflows to be tailored for the needs of every participant.
4. Easy to integrate
By default, an orchestration platform must include a secure and scalable integration framework that supports both event-driven and synchronous integration patterns, as well as the ability to map to different API formats and protocols. When it comes to personalized medicine, this is even more imperative as most calls to external systems must take place in real-time across a network of disparate systems that include ERP, MES, CTMS, Courier, EHR, and more.
Most of these integrations require synchronous calls from the user perspective. Yet, the preferred way to scale integrations and make them resilient to unexpected issues is to have the systems communicate asynchronously. The ability to scale across real-time messages to a multitude of external systems using various different protocols and formats is a therefore a challenge not unique to the personalized medicine but certainly much more fundamental to achieving scale in industry.
A proven approach, as adopted by other enterprise cloud-based platforms is to build a sophisticated integration framework into the core offering. Many such platforms embed open source integration frameworks that include enterprise service bus and API management platform.
5. Quick to implement
When a new therapy is launched commercially, manufacturers have to on-board hundreds of hospital sites around the globe, all with diverse systems, processes, and national and regional regulations that are constantly evolving. On top of that, many manufacturers are building geographically distributed facilities in an effort to more efficiently serve different regions, which will inevitably add pressure to capacity management and routing capabilities.
All of these factors mandate a platform that can be very quickly configured to meet the specific needs of each therapy and region. A significant part of any implementation project is the time taken in validating the deployment. New and innovative approaches are needed for validating such a highly configurable platform as validating all possible configurations is neither practical nor necessary. The platform has to be designed in such a way that each release of its software could be validated quickly. This requires approaching validations differently.
6. Secure, compliant, and always on
A unique requirement for autologous CAR-T platforms is that they have to be GMP and HIPAA compliant. As these therapies are being launched all over the world, other standards like GDPR become requirements, too.
Besides complying with the standards, security and privacy have to be baked into the software development processes and architecture of the platform. All code must go through security analysis and the system must later be tested by independent firms. Data for each therapy manufacturer has to be kept encrypted and isolated at rest, and protected by key management and accessibility policies. Role-based access control (RBAC) with configurable policies and High Availability (HA) have to be the core building blocks.
Add to that, all communications must be encrypted, logged, and monitored for malicious activity, and these are just a few of the high-level security, compliance, and HA requirements for personalized therapy platforms.
7. Supports continuous and durable innovation
New technologies or regulatory requirements can impact the workflows overnight. Cloud-based configurable platforms allow critical updates and continuous innovation to be delivered to all parties without compromising the specific configurations. This is radically different from a custom application that must be independently updated, forcing customizations to be re-implemented at an additional cost.
8. Provides rich business intelligence (BI) and configurable rules and notifications
The platform must provide a BI framework that includes ad-hoc reporting, real-time dashboards, and data export capabilities. The notification framework should be configurable and monitor the supply chain in real-time to notify the appropriate stakeholders when unexpected conditions occur or are predicted to occur.
Scheduling and logistics is an area that presents such a large number of possible permutations that it is not possible to model all of them as workflows. In such cases, the orchestration platform has to provide a rule-based system that uses machine learning to tweak its rules overtime. For example – a rule such as ‘Pick the manufacturing facility that provides the fastest cycle time’ is a deceptively simple rule. This rule requires the system to learn from historical data and then apply pre-configured rules to predict the best manufacturing site and the fastest route to get there. Then monitor that decision and adjust if needed.
Such a system can take some decisions itself but may still require humans to make the rest of the decisions. That’s where the system has to notify the stakeholders and assist them with analytics so that they can pick the right permutation at runtime.
9. Scales globally to support a large number of patients across many therapies
With just a handful of commercial therapies, the patient population is small. However, as more therapies are commercialized that target several different indications, the population of eligible patients would increase dramatically. The platform for personalized medicine has to soon support hundreds of thousands of patients across the world while providing an always-on, fast and consistent experience. This when combined with the fact that each manufacturer’s data must be kept isolated at rest introduces unique technical challenges. New cloud orchestration frameworks such as Kubernetes help scale each customer’s deployment separately while providing economies of scale.
Conclusion
Personalized medicine supply chain is the most complex, time-sensitive and critical supply chain in the history of medicine. It deserves an orchestration platform that is not just best of breed but is purpose built to meet the unique needs of this industry. We contend that the nine characteristics discussed in this article are the very foundations of such a platform. As such they should be part of the baseline criteria therapy manufacturers use when evaluating such platforms.
We hope you found this article useful. Feel free to contact me if have comments or would like to discuss further.