Oct 28
2020
Three Different Medical Device CE Markings and What They Mean
The CE mark we know and recognize today was originally an EC mark, but in 1993, this was replaced with the CE mark we use now, which is found in directive 93/68/EEC. This CE mark is abbreviated from the French ‘Conformité Européene’ and is usually displayed on medical devices or on the packaging of medical devices sold in the European Economic Area (EEA). This CE mark is a form of proof that the medical device meets certain EU requirements.
A CE marked medical device, such as a hearing aid or a pacemaker, indicates that it meets the health, safety and environmental standards of the EU, as well as all EU legislation. CE markings aren’t just used for the member states of the EEA but Turkey and Switzerland also use this certification which is applicable to medical devices regardless of whether manufacturing happened inside or outside of the EEA. Because of the wide usage of the CE marking, it’s important that you as a user understand the different types of CE markings you will encounter.
The CE mark for European Conformity

If a product is intended to be used for medical purposes, is a contraceptive or is used to clean a medical device, it is likely a medical device. This is something that the manufacturer decides at an early stage, and if the product fulfils the criteria in the Medical Device Regulation (MDR) it will be classified as a medical device and receive the CE mark.
In particular, medical devices must meet the general safety and performance requirements of the MDR. This in turn, means that they have to meet the requirements of various ISO and IEC standards for medical devices.
Manufacturers are only allowed to affix the CE mark on a medical device after meeting those requirements. This means that the CE mark tells you as a consumer that the product is safe to use.
The China Export CE marking
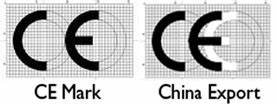 With so much manufactured in China, you may also encounter a China Export CE mark which does not provide any verifications of safety or performance for a medical device. This Chinese export mark looks very similar to the CE mark found on medical devices, but with the difference that it only informs you that the product was exported from China.
With so much manufactured in China, you may also encounter a China Export CE mark which does not provide any verifications of safety or performance for a medical device. This Chinese export mark looks very similar to the CE mark found on medical devices, but with the difference that it only informs you that the product was exported from China.
The difference between a real CE mark and a fake CE mark
The difference between the CE mark and the Chinese export mark can be difficult to spot. However, if you look closely, you will notice that the letters CE are much closer together on the China export marking. You can also check to make sure that the CE mark is legit, by checking the following:
- The letters CE are in the standard, recognised form
- If you reduce or enlarge the size of the CE marking, the letters CE must be in proportion to the standard version
- The CE mark is at least five mm, unless a larger minimum dimension is specified in the relevant directive
- The CE marking is placed on the product or its data plate. If this is not possible or not warranted because of the nature of the product, it will be placed on the packaging or the product documentation, preferably the instructions for use
- The CE mark is easily visible, readable and permanent
If you are unsure if the CE mark on medical device is a China export mark or not, you can always request a declaration of conformity from the manufacturer.
CE markings with a four-digit number
There is also one other kind of CE mark that you may find on class IIa or higher classes of medical devices. This CE marking is followed by a four-digit number, the NB number, which stands for Notified Body.
 Simply put, the NB number is an identification number that can help to identify which external regulatory body reviewed or approved the medical device before the CE mark was put on the product.
Simply put, the NB number is an identification number that can help to identify which external regulatory body reviewed or approved the medical device before the CE mark was put on the product.
The four- digit number is often found on the right side of the CE letters, or below them. You can find the associated notified body in the list of bodies notified under directive 93/42/EEC on the EU website.
Medical devices sold without CE markings
There are a few instances where you may encounter a product that seems to have the features of a medical device, but that does not carry the CE mark.
Certain products, like Garmin’s activity bracelets have functions such as pulse measurement that could be used for medical purposes. However, it is the manufacturer that decides if they want to label their product a medical device or not. Garmin has, for example, been very clear in its instructions for use, that this device is not intended to be used as a medical device, and as such it does not need to carry the CE mark as a medical device (however, there might be other applicable directives that requires it to be CE-marked).
If you are sure that a product is classified as a medical device, but it’s not marked with the CE symbol, then this could be due to one of two reasons. Certain devices are too small to fit a CE mark, such as tooth implants, or due to the nature of the medical device, it is difficult to put it on the product itself, such as a needle. In these cases, you will find the CE mark on the packaging and the documentation.
If you are unable to find the CE mark in the documentation or on the device’s packaging, this could mean that the manufacturer has omitted it due to negligence, or intentionally if the product has not met the necessary requirements.
Summary
The most important thing to remember when purchasing or using a medical device is that it should be safe to use and, as a user, you should use it for its intended purpose.
A medical device with a legitimate CE marking and documentation warrants that the device conforms to certain standards in the EU. If you are unsure of whether or not the CE mark on your medical device is fake or not, you should always contact the manufacturer and ask for a declaration of conformity.
Keep in mind that there are many items in daily life that are considered medical devices. Condoms, hearing aids, plasters and even glasses are medical devices and should carry the CE mark.