| “Every day, heroic nurses, doctors, and other healthcare workers are dedicating long hours to their patients. This means sacrificing time with their families and risking their very lives to care for coronavirus patients,” said CMS Administrator Seema Verma. “Front line healthcare providers need to be able to focus on patient care in the most flexible and innovative ways possible. This unprecedented temporary relaxation in regulation will help the healthcare system deal with patient surges by giving it tools and support to create non-traditional care sites and staff them quickly.”
Other temporary CMS waivers and rule changes dramatically lessen administrative burdens, knowing that front line providers will be operating with high volumes and under extraordinary system stresses.
CMS recently approved hundreds of waiver requests from healthcare providers, state governments, and state hospital associations in the following states: Ohio; Tennessee; Virginia; Missouri; Michigan; New Hampshire; Oregon; California; Washington; Illinois; Iowa; South Dakota; Texas; New Jersey; and North Carolina. With today’s announcement of blanket waivers, other states and providers do not need to apply for these waivers and can begin using the flexibilities immediately.
Administrator Verma added that she applauds the Mar. 23, 2020, pledge by America’s Health Insurance Plans (AHIP) to match CMS’s waivers for Medicare beneficiaries in areas where in-patient capacity is under strain. “It’s a terrific example of public-private partnership and will expand the impact of Medicare’s changes,” Verma said.
CMS’s temporary actions announced today empower local hospitals and healthcare systems to:
- Increase Hospital Capacity – CMS Hospitals Without Walls:
CMS will allow communities to take advantage of local ambulatory surgery centers that have canceled elective surgeries, per federal recommendations. Surgery centers can contract with local healthcare systems to provide hospital services, or they can enroll and bill as hospitals during the emergency declaration as long as they are not inconsistent with their State’s Emergency Preparedness or Pandemic Plan. The new flexibilities will also leverage these types of sites to decant services typically provided by hospitals such as cancer procedures, trauma surgeries and other essential surgeries.
CMS will now temporarily permit non-hospital buildings and spaces to be used for patient care and quarantine sites, provided that the location is approved by the State and ensures the safety and comfort of patients and staff. This will expand the capacity of communities to develop a system of care that safely treats patients without COVID-19, and isolate and treat patients with COVID-19.
CMS will also allow hospitals, laboratories, and other entities to perform tests for COVID-19 on people at home and in other community-based settings outside of the hospital. This will both increase access to testing and reduce risks of exposure. The new guidance allows healthcare systems, hospitals, and communities to set up testing sites exclusively for the purpose of identifying COVID-19-positive patients in a safe environment.
In addition, CMS will allow hospital emergency departments to test and screen patients for COVID-19 at drive-through and off-campus test sites.
During the public health emergency, ambulances can transport patients to a wider range of locations when other transportation is not medically appropriate. These destinations include community mental health centers, federally qualified health centers (FQHCs), physician’s offices, urgent care facilities, ambulatory surgery centers, and any locations furnishing dialysis services when an ESRD facility is not available.
Physician-owned hospitals can temporarily increase the number of their licensed beds, operating rooms, and procedure rooms. For example, a physician-owned hospital may temporarily convert observation beds to inpatient beds to accommodate patient surge during the public health emergency.
In addition, hospitals can bill for services provided outside their four walls. Emergency departments of hospitals can use telehealth services to quickly assess patients to determine the most appropriate site of care, freeing emergency space for those that need it most. New rules ensure that patients can be screened at alternate treatment and testing sites which are not subject to the Emergency Medical Labor and Treatment Act (EMTALA) as long as the national emergency remains in force. This will allow hospitals, psychiatric hospitals, and critical access hospitals (CAHs) to screen patients at a location offsite from the hospital’s campus to prevent the spread of COVID-19.
- Rapidly Expand the Healthcare Workforce:
Local private practice clinicians and their trained staff may be available for temporary employment since nonessential medical and surgical services are postponed during the public health emergency. CMS’s temporary requirements allow hospitals and healthcare systems to increase their workforce capacity by removing barriers for physicians, nurses, and other clinicians to be readily hired from the local community as well as those licensed from other states without violating Medicare rules.
These healthcare workers can then perform the functions they are qualified and licensed for, while awaiting completion of federal paperwork requirements.
CMS is issuing waivers so that hospitals can use other practitioners, such as physician assistants and nurse practitioners, to the fullest extent possible, in accordance with a state’s emergency preparedness or pandemic plan. These clinicians can perform services such as order tests and medications that may have previously required a physician’s order where this is permitted under state law.
CMS is waiving the requirements that a certified registered nurse anesthetist (CRNA) is under the supervision of a physician. This will allow CRNAs to function to the fullest extent allowed by the state, and free up physicians from the supervisory requirement and expand the capacity of both CRNAs and physicians.
CMS also is issuing a blanket waiver to allow hospitals to provide benefits and support to their medical staffs, such as multiple daily meals, laundry service for personal clothing, or child care services while the physicians and other staff are at the hospital and engaging in activities that benefit the hospital and its patients.
CMS will also allow healthcare providers (clinicians, hospitals and other institutional providers, and suppliers) to enroll in Medicare temporarily to provide care during the public health emergency.
- Put Patients over Paperwork:
CMS is temporarily eliminating paperwork requirements and allowing clinicians to spend more time with patients. Medicare will now cover respiratory-related devices and equipment for any medical reason determined by clinicians so that patients can get the care they need; previously Medicare only covered them under certain circumstances.
During the public health emergency, hospitals will not be required to have written policies on processes and visitation of patients who are in COVID-19 isolation. Hospitals will also have more time to provide patients a copy of their medical record.
CMS is providing temporary relief from many audit and reporting requirements so that providers, healthcare facilities, Medicare Advantage health plans, Medicare Part D prescription drug plans, and states can focus on providing needed care to Medicare and Medicaid beneficiaries affected by COVID-19.
This is being done by extending reporting deadlines and suspending documentation requests which would take time away from patient care.
- Further Promote Telehealth in Medicare:
Building on prior action to expand reimbursement for telehealth services to Medicare beneficiaries, CMS will now allow for more than 80 additional services to be furnished via telehealth. During the public health emergencies, individuals can use interactive apps with audio and video capabilities to visit with their clinician for an even broader range of services. Providers also can evaluate beneficiaries who have audio phones only.
These temporary changes will ensure that patients have access to physicians and other providers while remaining safely at home.
Providers can bill for telehealth visits at the same rate as in-person visits. Telehealth visits include emergency department visits, initial nursing facility and discharge visits, home visits, and therapy services, which must be provided by a clinician that is allowed to provide telehealth. New as well as established patients now may stay at home and have a telehealth visit with their provider.
CMS is allowing telehealth to fulfill many face-to-face visit requirements for clinicians to see their patients in inpatient rehabilitation facilities, hospice and home health.
CMS is making it clear that clinicians can provide remote patient monitoring services to patients with acute and chronic conditions, and can be provided for patients with only one disease. For example, remote patient monitoring can be used to monitor a patient’s oxygen saturation levels using pulse oximetry.
In addition, CMS is allowing physicians to supervise their clinical staff using virtual technologies when appropriate, instead of requiring in-person presence.
For additional background information on the waivers and rule changes, go to: https://www.cms.gov/newsroom/fact-sheets/additional-backgroundsweeping-regulatory-changes-help-us-healthcare-system-address-covid-19-patient
For more information on the COVID-19 waivers and guidance, and the Interim Final Rule, please go to the CMS COVID-19 flexibilities webpage: https://www.cms.gov/about-cms/emergency-preparedness-response-operations/current-emergencies/coronavirus-waivers.
These actions, and earlier CMS actions in response to COVID-19, are part of the ongoing White House Coronavirus Task Force efforts. To keep up with the important work the Task Force is doing in response to COVID-19, visit www.coronavirus.gov. For a complete and updated list of CMS actions, and other information specific to CMS, please visit the Current Emergencies Website. |

 The Trump administration temporarily changed the policy for Centers for Medicare & Medicaid Services (CMS) allowing healthcare providers to have phone-only visits with patients, which is a major breakthrough in the industry and one that was not allowed before.
The Trump administration temporarily changed the policy for Centers for Medicare & Medicaid Services (CMS) allowing healthcare providers to have phone-only visits with patients, which is a major breakthrough in the industry and one that was not allowed before.

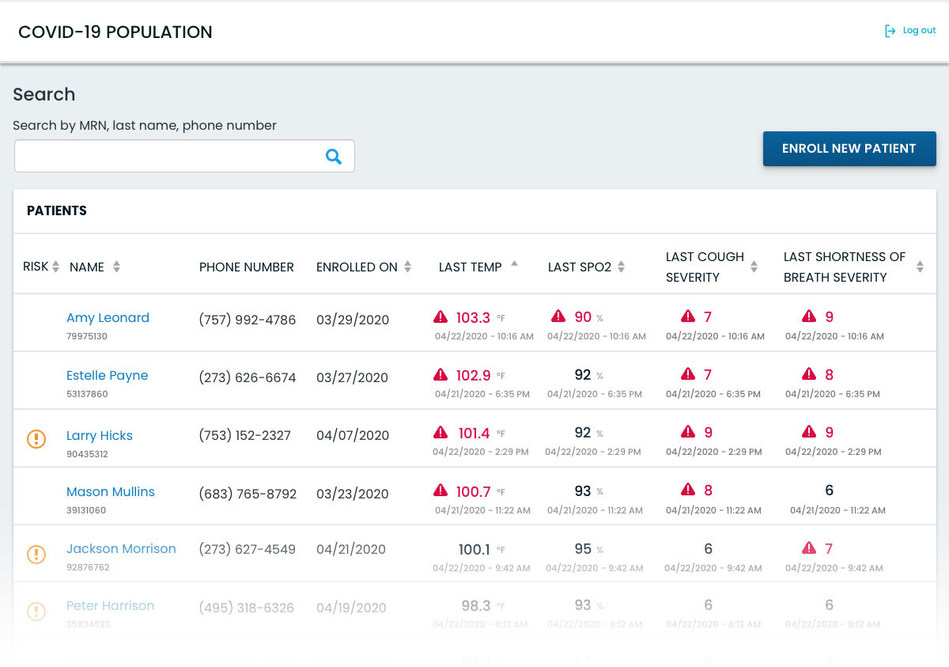
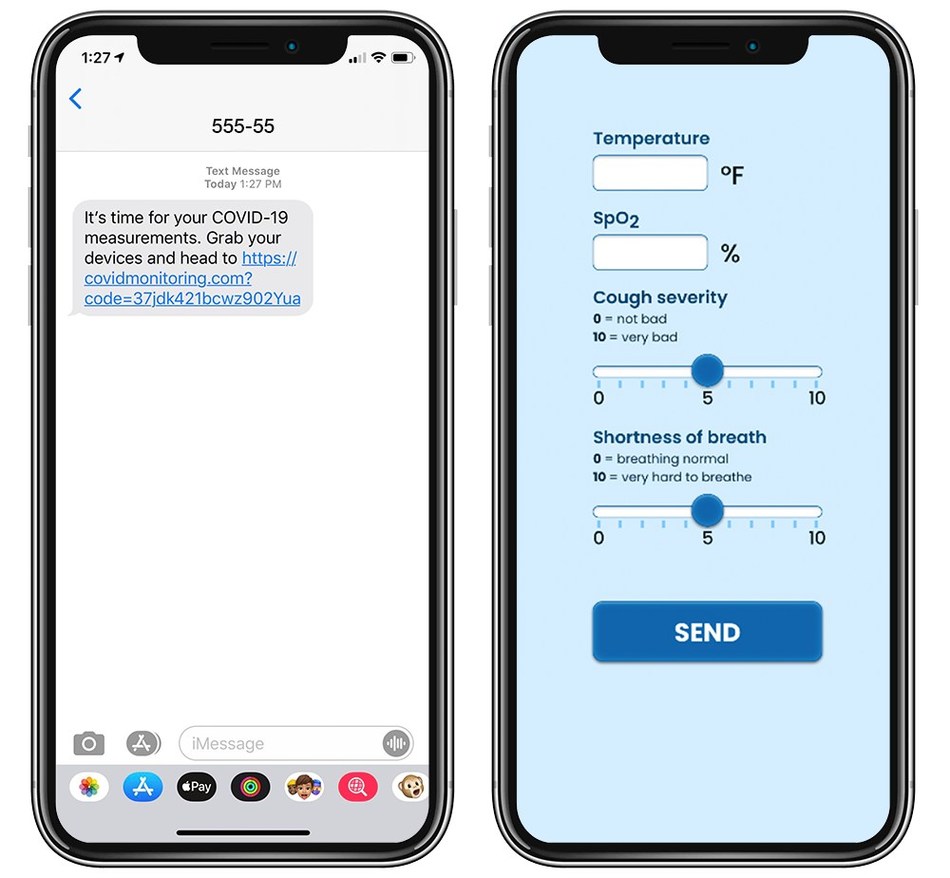
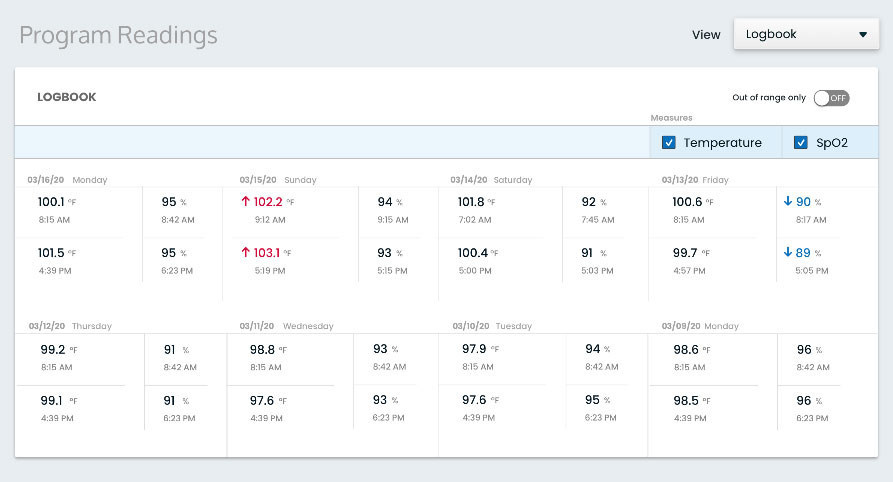
 Kathryn Whitney, MSc, director of thematic analysis at GlobalData, said: “Prior to the COVID-19 crisis, telemedicine had never reached its full potential in the US, with several barriers preventing its widespread uptake. These include lack of reimbursement and restrictions affecting access for rural populations, general lack of awareness of these services, and the desire of the sick to see their physician in person.”
Kathryn Whitney, MSc, director of thematic analysis at GlobalData, said: “Prior to the COVID-19 crisis, telemedicine had never reached its full potential in the US, with several barriers preventing its widespread uptake. These include lack of reimbursement and restrictions affecting access for rural populations, general lack of awareness of these services, and the desire of the sick to see their physician in person.”